The Molecular Formulas of Diatomic Bromine Chlorine Fluorine
Br 2 H 2 O HBr HBrO. Upload files PDF JPG GIF PNG TXT Word Excel Powerpoint file formats supported 02 File Limit The compound BrCl can decompose into Bry and Cly as represented by the balanced chemical equation below.
3 3 Molecules And Chemical Nomenclature Chemistry Libretexts
What are three examples of a diatomic element.

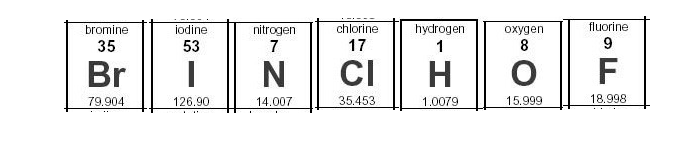
. Their molecular formulas would be written as H 2 N 2 O 2 How many diatomic elements are there at room temperature. Fluorine is a chemical element with the symbol F and atomic number 9. The molecular formulas of diatomic bromine chlorine fluorine and iodine are written below.
2 P 6 H 2 O 3 B r 2 6 H B r 2 H 3 P O 3. Chemical Engineering questions and answers 2. The molecules of hydrogen oxygen and nitrogen are composed of molecules that contain two atoms each called diatomic molecules and thus have the molecular formulas H.
It means that these elements can form a molecular element. Halogens have diatomic molecules. The compound BrCl can decompose into Br 2 and Cl 2 as represented by the balanced chemical equation below.
How do you remember the diatomic elements. A The molecular formulas of diatomic bromine chlorine fluorine and iodine are written below. This is because only five elements form stable diatomic molecules at standard temperature and pressure.
Chlorine Gas Formula Take h 2 hydrogen gas thats also a diatomic element like chlorine. Hydrogen bromide is a colourless gas at room temperature like all the hydrogen halides apart from hydrogen fluoride. Bromine dissolves in water forming hydrogen bromide and Hypobromous acid.
Circle the formula of the molecule that has the longest bond length. HOFBrINCl stands for Hydrogen Oxygen Fluorine Bromine Iodine Nitrogen Chlorine diatomic elements What is a molecular formula in chemistry. Used in gold mining extraction processes and in oil- and gas-well drilling.
Among the elements fluorine ranks 24th in universal abundance and. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas. The compound BrCl can decompose into Br 2 and Cl 2 as represented by the balanced chemical equation below.
As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium. What are the chemical formulas for each of the halogen molecules. As a result the elements are chemically joined together for example fluorine is formed as F chlorine as Cl bromine as Br and iodine as I.
The gases hydrogen nitrogen oxygen fluorine and chlorine. Uses of Diatomic Bromine Br 2. Compounds with 32 bromine are used in textile coatings spray-bonded nonwovens adhesives.
What Is The Diatomic Molecule Formula. View molecular formula for fluorine in WISER. From the given choices choice d iodine will be the answer since it can exist as a diatomic molecule molecular element.
Hydrogen nitrogen oxygen fluorine chlorine bromine and iodine form homo-nuclear diatomic molecules. Circle the formula of the molecule that has the longest bond length. Brinclhof Br I N Cl H O F where Br bromine I Iodine.
Fluorine F2 Chlorine Cl2 Iodine I2 Bromine Br2 Why are there only 7 diatomic molecules. Definition of molecular formula. Circle the formula of the molecule that has the longest bond length.
In the laboratory it is produced by the reaction of Bromine with red phosphorus. H 3 P O 3 H 2 O B r 2 2 H B r H 3 P O 4. Answer the following questions relating to the chemistry of the halogens.
The chemical equation is given below. Br2 Cl2 F2 I2. A The molecular formulas of diatomic bromine chlorine fluorine and iodine are written below.
F2 Cl2 I2 Br2 At2. Answer the following questions relating to the chemistry of the halogens. No water contains three atoms which include two hydrogens and one oxygen atom.
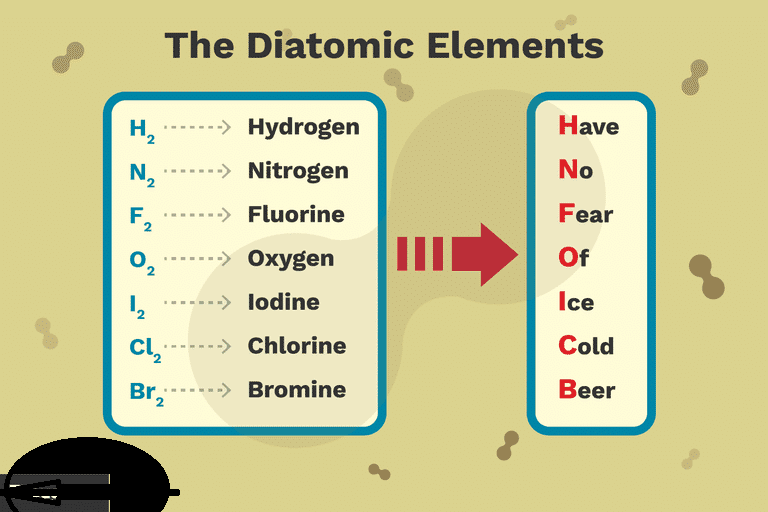
The elements found as diatomic molecules are hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine F element 9 chlorine Cl element 17 bromine Br element 35 and iodine I element 53. Br 2 Cl 2 F 2 I 2 b. Justify your choice in terms of atomic structure.
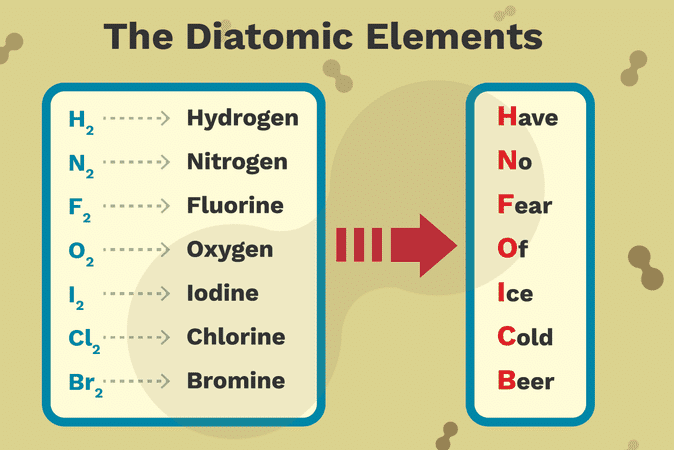
In total there are seven diatomic elements which are mentioned below HydrogenH 2 NitrogenN 2 OxygenO 2 BromineB 2 IodineI 2 ChlorideCl 2 fluorineF 2. 2 BrCl g Br 2 g Cl 2 g H 16 kJmol rxn. The formula of Fluorine is F and being highly reactive it exists as F2.
There are 7 of these diatomic elements and the easiest way I find to remember them is to use the mnemonic device Dr. Justify your choice in terms of atomic structure. Common diatomic molecules include hydrogen H 2 nitrogen N 2 oxygen O 2 and carbon monoxide CO.
Some sources will say there are five diatomic elements rather than seven. 2 BrCl9 Bra 9 Cl 9 AH 16 kJmolen Type here to search о я е да ENG US 4 DELL 3 of The compound BrCl can decompose into Bry and Cly as represented. The 7 diatomic elements are hydrogen H nitrogen N oxygen O fluorine F chlorine Cl bromine Br and iodine I.
A chemical formula that gives the total number of atoms of each element in each molecule of a substance compare structural formula. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together because they are extremely unstable alone due to their almost full valence shell. 2Br Cl g Br 2 g Cl 2 g ΔH16kJ mol rxn A 0100 mole sample of pure BrCl g is placed in a previously evacuated rigid 200L container at 298 K.
The intermolecular interaction in liquid bromine are London force while in BrCl are both dipole - dipole forces and London forces. The elements that can exist as diatomic molecules are hydrogen nitrogen fluorine bromine chlorine oxygen and iodine. Fluorine is a diatomic halogenfound as F2 Answer.
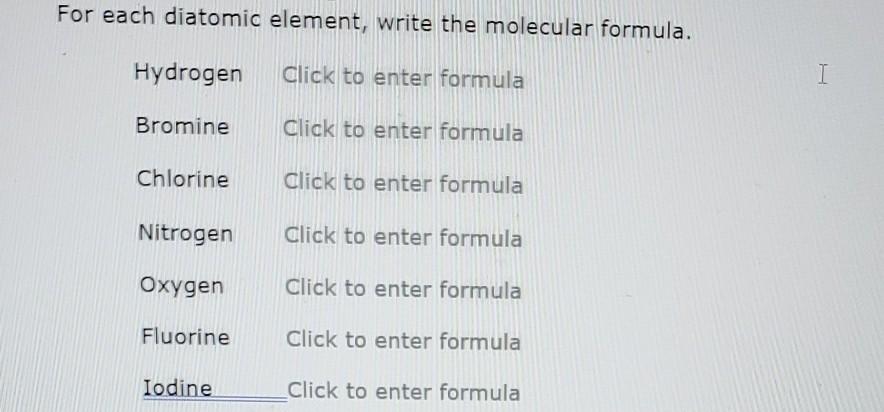
Solved For Each Diatomic Element Write The Molecular Chegg Com
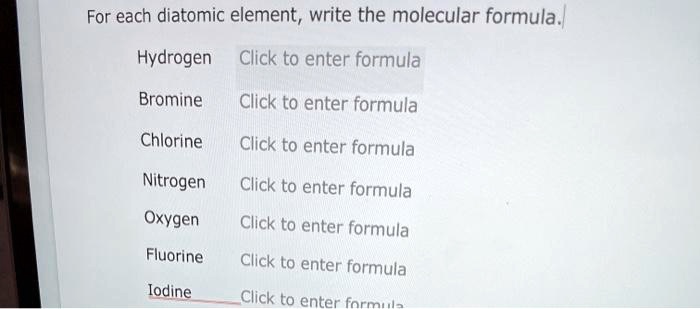
Solved For Each Diatomic Element Write The Molecular Formula Hydrogen Click To Enter Formula Bromine Click To Enter Formula Chlorine Click To Enter Formula Nitrogen Click To Enter Formula Oxygen Click To

Units 2 3 4 Frq Docx Answer The Following Questions Relating To The Chemistry Of The Halogens A The Molecular Formulas Of Diatomic Bromine Course Hero

Question Video Recalling The Type Of Bonding In Diatomic Halogens Nagwa

Chem Docx 2 Answer The Following Questions Relating To The Chemistry Of The Halogens A The Molecular Formulas Of Diatomic Bromine Chlorine Course Hero

Super Common Mistake Diatomic Elements Youtube

What Are The 7 Diatomic Elements Howstuffworks
Diatomic Elements Best Definition Example More Get Education Bee
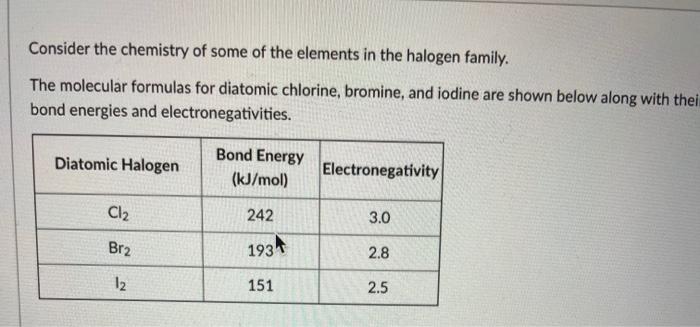
Solved Consider The Chemistry Of Some Of The Elements In The Chegg Com

Atomic Radius Measurements Of Diatomic Molecules Stock Vector Illustration Of Hydrogen Material 192485256
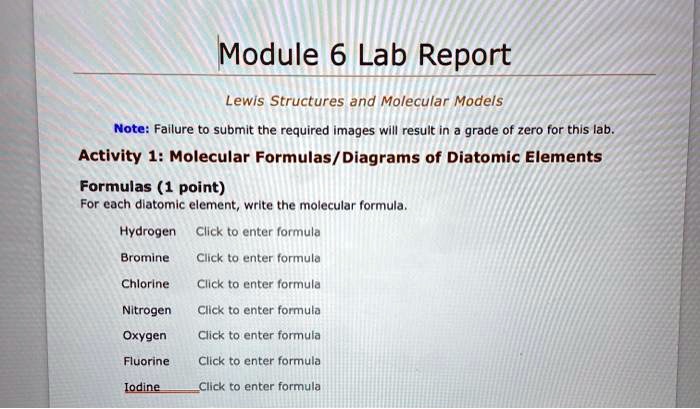
Solved Module 6 Lab Report Lewis Structures And Molecular Models Note Failure To Submit The Required Images Will Result In Grade Of Zero For This Lab Activity 1 Molecular Formulas Diagrams Of

2 Answer The Following Questions Relating To The Chegg Com
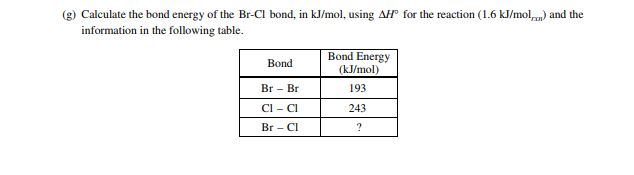
2 Answer The Following Questions Relating To The Chegg Com
What Is A Diatomic Element Education Is Around

Chem Docx 2 Answer The Following Questions Relating To The Chemistry Of The Halogens A The Molecular Formulas Of Diatomic Bromine Chlorine Course Hero
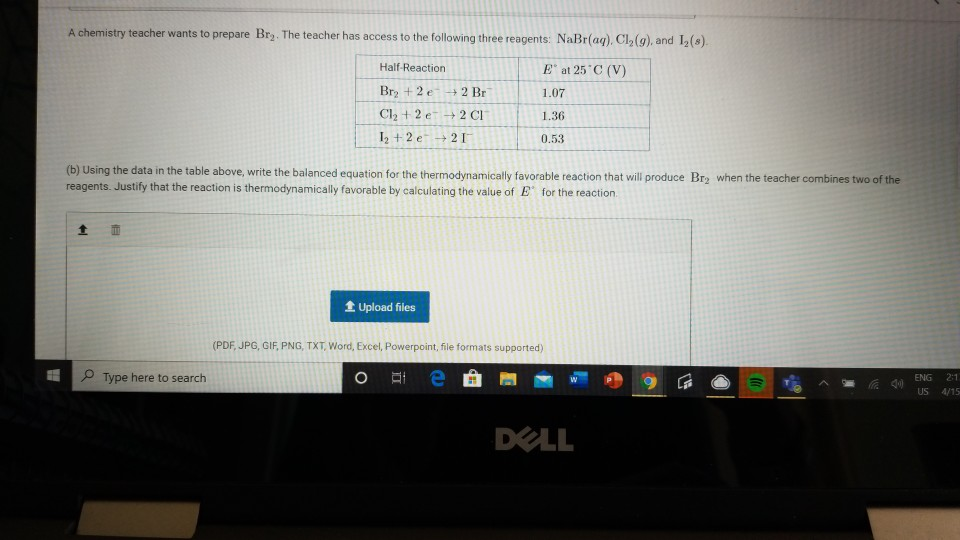
Solved Question 3 For Parts Of The Free Response Question Chegg Com

Diagram Explaining Atomic Radius Using Diatomic Molecules Oxygen Posters For The Wall Posters Water State Physical Myloview Com

Chem Docx 2 Answer The Following Questions Relating To The Chemistry Of The Halogens A The Molecular Formulas Of Diatomic Bromine Chlorine Course Hero
Comments
Post a Comment